Coronavirus COVID-19 is highly contagious and has caused a global pandemic. Early detection plays an important role in disease diagnosis, prevention, and treatment. RT-PCR is the most popular test to directly detect this virus. Recently, this test has been developed and manufactured by the US. Center for Disease Control and Prevention, Respiratory Virus Branch, Division of Viral Diseases. This testing technology has been shared with private companies for manufacturing purposes in response to the outbreak of the coronavirus disease.
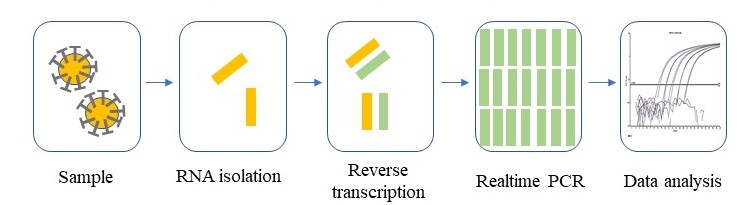
We have created a basic outline of the CDC testing scheme as above. Each step is critical for the success of the test.
- Sampling method: A validated clinical sampling method needs to be used to ensure the right amount of virus is taken for testing.
- RNA isolation method: An efficient protocol for isolation of RNA enables the effective isolation of RNAs from coronavirus present in the sample.
- Reverse transcription reaction: This reaction helps to produce complimentary DNA products from the viral RNA. A DNA ‘copy’ of the viral RNA is necessary for the PCR test to function.
- Realtime PCR: The small amount of DNA molecules can be amplified via Realtime PCR to produce sufficient DNA products for detection on a Realtime PCR instrument.
- Data analysis: The Realtime PCR results will then be compared with standards and analyzed to obtain quantifiable and accurate results.
In general, a PCR testing process may take hours to complete, but it can analyze up to a hundred samples for one test run. This large volume testing methodology will significantly save time and costs. Keep in mind, molecular diagnostics expertise is required in analytical labs performing these critical tests to ensure quality data is generated and accurately interpreted. In addition, due to the nature of the coronavirus disease, only a Biosafety Level 3 lab is qualified to run diagnostic testing for coronavirus COVID-19. At Cicadea Biotech, we provide consulting services or technical support for coronavirus testing.
